This study will help us find out.#
Learn more about the Vista study and discover if participation may be an option for you.
Thank you for your interest in the Vista study#
This study will investigate the safety and efficacy of a new investigational medicine in adults who are living with obesity or excess weight with at least one weight-related medical condition (such as cardiovascular disease (CVD) or high blood pressure).
This pamphlet should be used alongside the information you get from the study team to answer any questions you might have about the study.
What is a clinical trial?#
It’s a type of medical research. In clinical trials, volunteers help researchers find out how new treatments work, and if they can be used safely. Sometimes the volunteers in clinical trials are healthy individuals. Other times, like for this study, the volunteers have a particular health condition related to the treatment that is being studied.
Volunteer participants are essential for this important research to happen.#
What is Vista?#
Vista is a clinical trial looking at different doses of the investigational treatment, AZD5004, to see which doses work best to help people lose weight. The study will also find out more about the safety of AZD5004 and how the body responds to it.
AZD5004 is an investigational treatment. This means it has not been fully tested and has not been approved by any health authority, except for use in research studies like this.
The data that we collect from Vista will help researchers plan future studies to find out more about the study treatment.
How does AZD5004 work?
AZD5004 works like a natural hormone called GLP-1, which is released by our small intestine when we eat. GLP-1 sends signals that let our bodies know when we are full, slows down the speed of the stomach emptying, and helps to control blood sugar levels. Because this type of medication makes you feel less hungry and fuller for a longer time, it may also help with weight loss.
How will I take the study treatment?
In this study, AZD5004 will be taken as a tablet. Currently, the approved GLP-1 medications used to treat obesity in adults are in the form of injectables. However, researchers think that it will be easier for some people to take tablets than have injections.
What is involved in taking part?
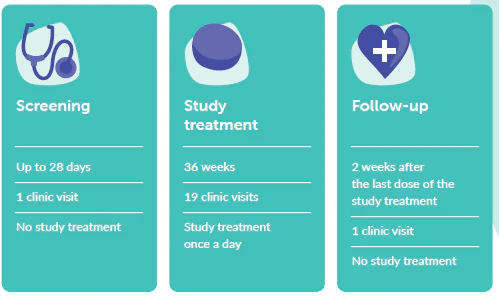
You will be on the Vista study for up to 10 months (approximately 42 weeks). During this time, you will attend 21 clinic visits.
Vista is split into 3 parts:
Treatment groups
Everyone on the study will be randomly assigned to receive 1 of 5 different doses of AZD5004 or a placebo.
Placebos look like the investigational treatment and are taken the same way, but they do not contain any active medicine. They help us to understand the effects of the investigational treatment.
Neither you nor your study doctor will know which treatment group you have been assigned to. This is called a double-blind study.
If your doctor needs to know your treatment group, they can find out quickly and easily.
More people will receive AZD5004 than the placebo
During the study, you will be asked to#
- Come into the clinic for regular clinic visits. Each visit may vary in length depending on the tests and procedures to be done
- Bring your empty tablet bottles and any unused study treatment with you to each visit
- Have study tests and assessments
- Including regular blood and urine samples and electrocardiograms (ECGs)
- Take the study treatment once a day, as instructed
- Complete a Dosing Diary daily and bring it with you to every study visit
- Complete a Symptom Diary with any side effects you experience and bring it with you to every study visit
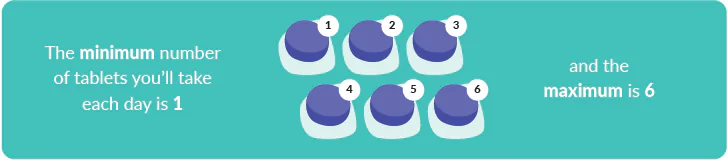
- How many tablets will I need to take? The purpose of the Vista study is to discover the dose of AZD5004 that most effectively manages weight loss.
- The number of tablets you’ll take will depend on which study group you’re in. It may also change over time if the study team need to increase or decrease your dose
Is there anything else I should know?#
- All study-related visits, tests, and study treatments will be provided at no cost to you
- If applicable, you may be reimbursed for some expenses while you take part (for example, travel or parking)
- As with all medicines, the study treatment may have some side effects. Your study team can discuss this with you further to help you understand the benefits and risks of taking part
- Your study treatment must be taken at the same time every day
- You need to fast before some study visits. This means you will be asked to avoid food or drink (except plain water) before the study visit
- You cannot take certain medications, including some weight loss medications, while you are on the Vista study. The study team will tell you more about this
If you still have questions#
Please speak to a member of the study team if you still have any questions.
Here are some topics that you might want to think about before committing to taking part in the Vista study:
- Taking the study treatment every day
- How you feel about the possibility of being assigned to the placebo treatment group
- The tests and assessments you may need
- Possible side effects from the study treatment
- The time commitment and schedule of clinic visits over a 10-month period
- What diet and lifestyle changes you can make alongside receiving the study treatment
Are you interested in taking part?#
Speak to a member of the study team if you’d like more information on how to join. 786-772-0510