Volunteers
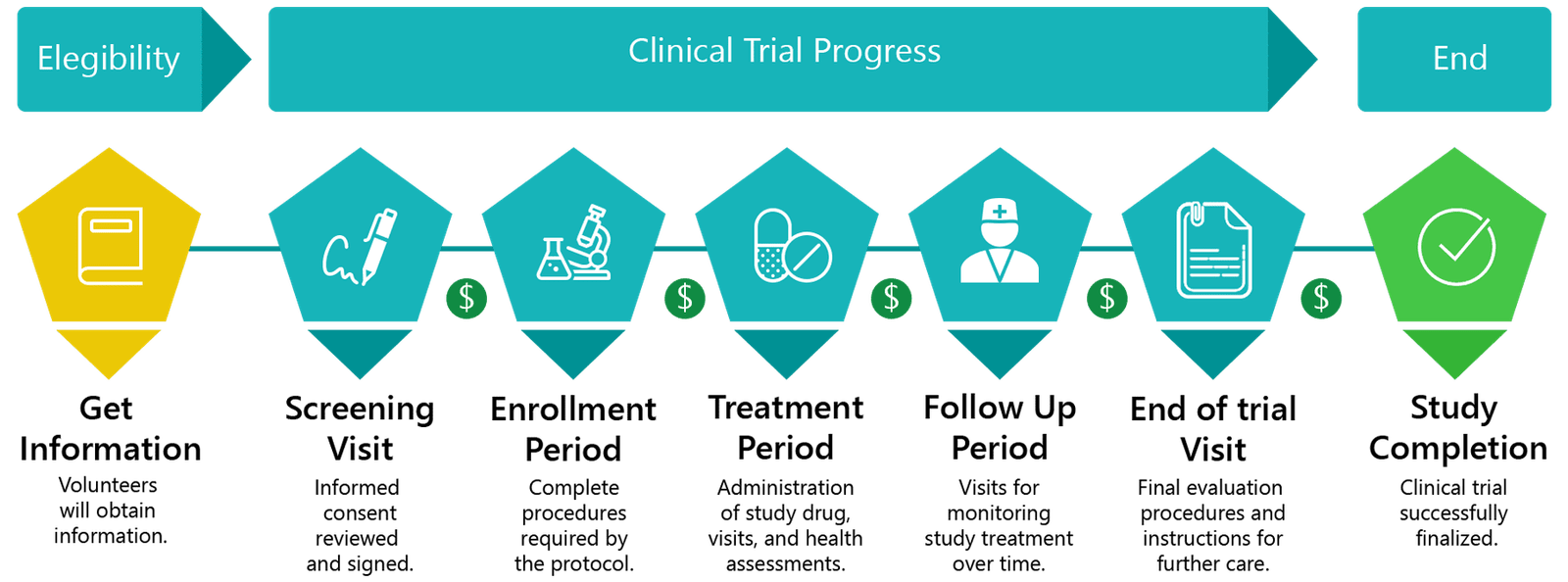
Brief overview of study progress
FAQ

A clinical trial is a research study in human volunteers intended to answer particular health questions. Clinical trials are conducted to develop new medications or treatments that can improve health. After a medication or treatment is found to be safe and effective, it may be approved by the FDA and then, it will be available for population. No treatment may reach clinical testing unless there is evidence that there might be an improvement over current therapies.
Medical treatment cannot improve without research and volunteers. Everyone would like a successful treatment available when diagnosed with an illness. By volunteering for clinical trials you can help advance knowledge about future treatments that someday you and a loved one may benefit from. In a research study in clinical trials you have the ability to take an active role in your own health, access possible investigational medications or treatments before they are widely available, and help others by contributing to the participants in return for their time and travel.


All clinical trials have guidelines about who can get into a specific study. The factors that permit someone to participate in a clinical trial are called “inclusion criteria” and those that forbid someone from participating are called “exclusion criteria”. These criteria are based on such factors as age, gender, the type and stage of a disease, previous treatment history, and other medical conditions. In order to join clinical trials, a participant must meet the requirements for the study. Some research studies seek participants with illnesses or conditions to be studied in the clinical trial, while others need healthy participants. Inclusion and exclusion criteria are used to identify proper participants and keep them safe. The criteria help ensure that research questions will be answered properly.
Besides playing an active role in your own health care and helping others by contributing to medical research, by participating in clinical trials you can also enjoy benefits like:
Have access to new medical treatments or medications that are not available in the market or in medical clinics.
Obtain medical care at top health care facilities during the trial.
If you qualify you may receive a stipend to help compensate for time and travel.
Satisfaction of taking part in medication research that can help others
No-cost study-related care
No health insurance is required to participate in Clinical Trials.

All study-related procedures such as Physician Evaluations, Lab tests, EKGs, Radiological imaging, and study medication are provided at no cost to you, plus economical compensation for your time and travel (as applicable). Health Insurance is not necessary.

