
Learn more about our COVID-19 vaccine study for healthy adults and adolescents 12 years and older.

Learn more about our COVID-19 vaccine study for healthy adults and adolescents 12 years and older.

Learn more about Pfizer’s research study for an investigational COVID-19 and flu combination vaccine.
Join in the fight against Covid/Flu Viruses
Are you eligible for our vaccine clinical research studies?
• No health insurance required
• Compensation for time and travel for qualified patients
Call Today!
786-772-0510
IMA Clinical Research is seeking healthy adults for our
groundbreaking vaccine research studies.
We are hoping to find new and effective options to fight
against the Covid and Influenza viruses.
• Ages 18 – 64
• In Generally Good Health
• Potentially discover new and effective treatment options
• Compensation provided for time and travel (for qualified
patients)
• No health insurance required
Together we can make a real difference in medical advancements. Join us on our journey toward improved immunity!
To learn more about our studies and how to participate:
Call us

This study will help us learn if different investigational mRNA combination vaccines are safe and can help the body produce antibodies which may help fight off different strains of flu as well as COVID-19.

Learn more about participating in the Duo P301 Trial for an investigational combination vaccine aimed at preventing respiratory illness caused by both seasonal influenza (flu) and COVID-19 in adults 50 years of age and older

Advances in science are changing how we fight respiratory infections. Introducing Pfizer’s research study for an investigational combination vaccine that may protect against COVID-19 and RSV in one convenient shot.

You have the power to advance research that may change the future of seasonal influenza (flu) with 1 injection. Learn more about participating in the Ignite P303 Trial for an investigational vaccine aimed at preventing seasonal flu infection.

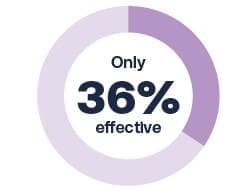
Seasonal flu vaccines during the 2021-2022 flu season were2:
A messenger RNA (mRNA) vaccine may offer broader protection by responding to changes in seasonal flu strains more quickly and creating stronger immune responses3.
The Ignite P303 Trial will look at the safety and immune response of an mRNA-based investigational vaccine aimed at preventing seasonal flu in adults aged 18 and older.
Who Can Join?
This clinical trial is looking for adult participants. To join, you must be:
⚪ 18 years of age or older
⚪ In good health
⚪ Not pregnant or planning on becoming pregnant for at least 3 months following your vaccine visit
Prior to enrolling in this trial, you should:
⚪ Not have received a seasonal flu vaccine in the past 5 months
⚪ Not have had a confirmed flu infection within 5 months
References:
1. Influenza (seasonal): ask the expert: influenza Q&A. World Health Organization. Published November 6, 2018. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
2. Price AM, Flannery B, Talbot HK, et al. Influenza vaccine effectiveness against influenza A(H3N2)-related illness in the United States during the 2021-2022influenza season. Clin Infect Dis. 2022;ciac941. doi:10.1093/cid/ciac941
3. Rockman S, Laurie KL, Parkes S, Wheatley A, Barr IG. New technologies for influenza vaccines. Microorganisms. 2020;8(11):1745.